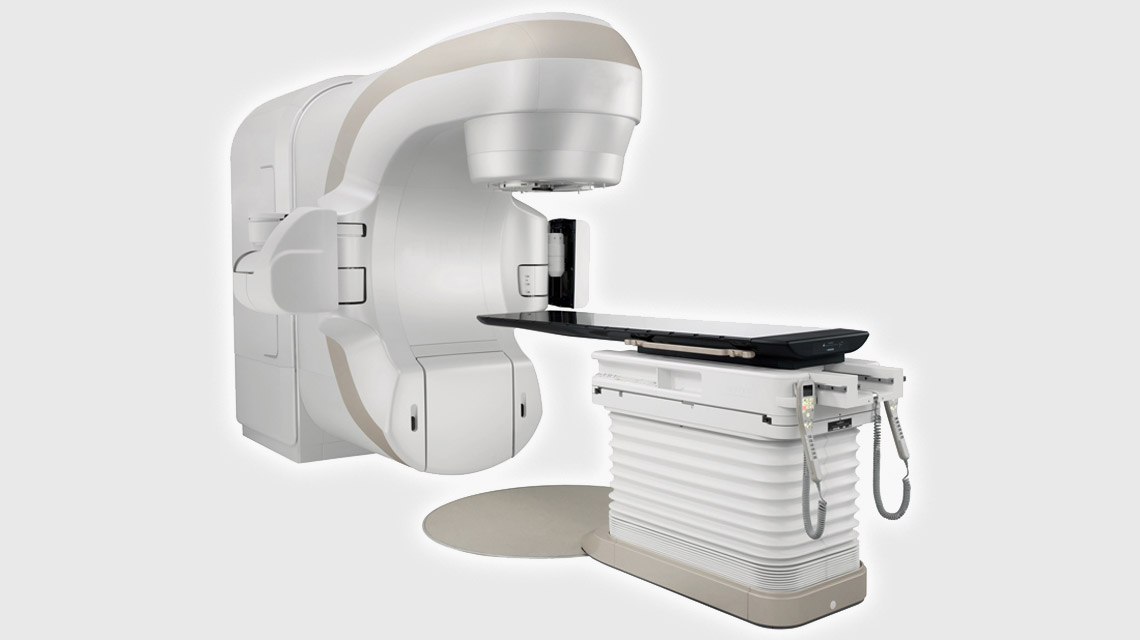
Linear accelerators (linacs) have become one of the most important technologies used in radiation therapy for cancer treatment. These machines use microwave technology and high-energy electromagnetic fields to generate and precisely deliver targeted beams of radiation to tumors in patients. In this article, we will explore the history and evolution of linac technology, how they work, their widespread applications in modern cancer care, and ongoing research enhancements.
The History of Linear Accelerator Development
The invention of the linear particle accelerator dates back to 1927 by American physicist Robert J. Van de Graaff. His original device accelerated charged particles using static electricity, laying the foundation for future developments. However, it was not until the 1950s that linacs first began emerging as clinically useful radiation therapy tools. Pioneering work by researchers like William Hanson at Stanford University led to the design of the first medical electron accelerator or linac in 1954. This marked the start of using externally generated electron beams for radiotherapy applications.
Over subsequent decades, linac technology advanced rapidly. The 1960s saw major upgrades in power and reliability. Variants capable of generating higher energy X-ray or proton beams were also produced. By the 1970s and 1980s, modern medical linear accelerator emerged with sophisticated microprocessor controls and imaging systems integrated. This allowed accurate targeting and beam delivery that was more precise than earlier radiology methods like radioactive isotopes or obsolete machines. Today’s state-of-the-art linacs are the direct descendants of these foundational innovations dating back 70 years.
How Linear Accelerators Generate and Deliver Radiation Beams
At their core, all modern linacs use microwave technology to generate high-energy particle beams for radiation therapy. The basic components and process are as follows:
– Electron Gun: Emits a steady stream of low-energy electrons which are accelerated down the waveguide.
– Waveguide: Housed in an evacuated chamber, it contains traveling electromagnetic waves that accelerate the electrons to nearly light speed.
– Target: As electrons smash into a heavy metal target like tungsten, their impact produces either X-rays or proton beams depending on the model.
– Gantry: A rotating ring houses the linear accelerator and particle source. It enables the radiation beam to be precisely aimed at the tumor from various angles around the patient on the treatment table.
– Collimators: Magnetic fields and heavy metal jaws are used to sculpt the particle beam into the desired shape and limit radiation exposure to healthy tissues beyond the tumor boundaries.
– Imaging and Controls: Onboard CT imaging and computer planning allow clinicians to verify patient positioning and accurately align beams prior to each fractionated treatment session.
With the beam properly generated and aligned using these core components, clinicians can then deliver precisely modulated doses of radiation to cancers in patients over multiple brief sessions, slowly destroying malignant cells while sparing surrounding healthy tissues.
The Range of Modern Clinical Applications
Today there are an estimated 20,000 linear accelerators installed worldwide, accounting for the vast majority of external beam radiation procedures. Due to their precision abilities, linacs are indispensable technology for a wide variety of clinical scenarios:
– Early Stage Cancers: As a non-invasive alternative or addition to surgery, linacs can often cure localized cancers with a moderate number of carefully calibrated treatment sessions. This includes tumors of the prostate, breast, lung and more.
– Advanced/Metastatic Cases: Even for cancers that have spread to distant sites, palliative linac therapies can still effectively relieve pain and symptoms from metastatic bone lesions for example. This improves quality of life.
– Pediatrics: Children with cancers amenable to radiation therapy can avoid invasive surgery with linac treatments tailored to their smaller bodies. Doses are much more accurately confined to the tumor.
– Integration with Imaging: The use of onboard CT and PET imaging allows for image-guided radiotherapy, helping ensure doses are optimized based on daily anatomical variations and tumor location verification before each fraction.
– Proton Beam Therapy: Some clinics now offer proton linacs as a more advanced option than traditional X-rays. Protons deposit most of their energy at a specified depth, minimizing exit dose beyond the target.
Ongoing Research and Next Generation Advances
In parallel with helping treat millions of patients, linac developers and medical physicists continue striving to enhance capabilities. Areas of active investigation include:
– FLASH Radiotherapy: Research suggests ultra-high dose rates may differentially spare healthy tissues from damage compared to conventional fractions. This could yield safer hypofractionated schedules.
-MRI-Linac Fusion: Combining the soft tissue imaging abilities of MRI with radiotherapy will provide superior real-time tumor tracking and adaptive treatments compensating for daily anatomical changes.
-Proton Therapy Accessibility: As costs decrease through innovations, proton linacs may become widely available alternatives to X-rays for particular pediatric cancers or those adjacent to critical structures.
– FLASH-SRT: Ultra-high dose per pulse stereotactic radiosurgery techniques could offer non-invasive ablative alternatives to neurosurgery for some brain and spine indications.
In summary, over 70 years since their inception, linear accelerators have become essential drivers of progress in modern cancer radiotherapy. Through continual technological refinements, they will likely remain the preeminent radiation delivery platform for years to come.
*Note: