A groundbreaking study led by Professors Hongtae Kim and JaYil Lee from the Department of Biological Sciences, in collaboration with researchers from the Catholic University of Korea, sheds new light on the critical role of mutated DDX41 protein in the pathogenesis of Myelodysplastic Syndrome (MDS), a form of blood cancer. The research offers novel insights into the complex relationship between genomic instability and the development of leukemia, potentially paving the way for new therapeutic targets for MDS treatment.
MDS is characterized by impaired production of normal blood cells in hematopoietic stem cells, resulting in a decrease in peripheral blood cell count. If left untreated, MDS can progress to acute myeloid leukemia (AML), particularly affecting male patients over the age of 65.
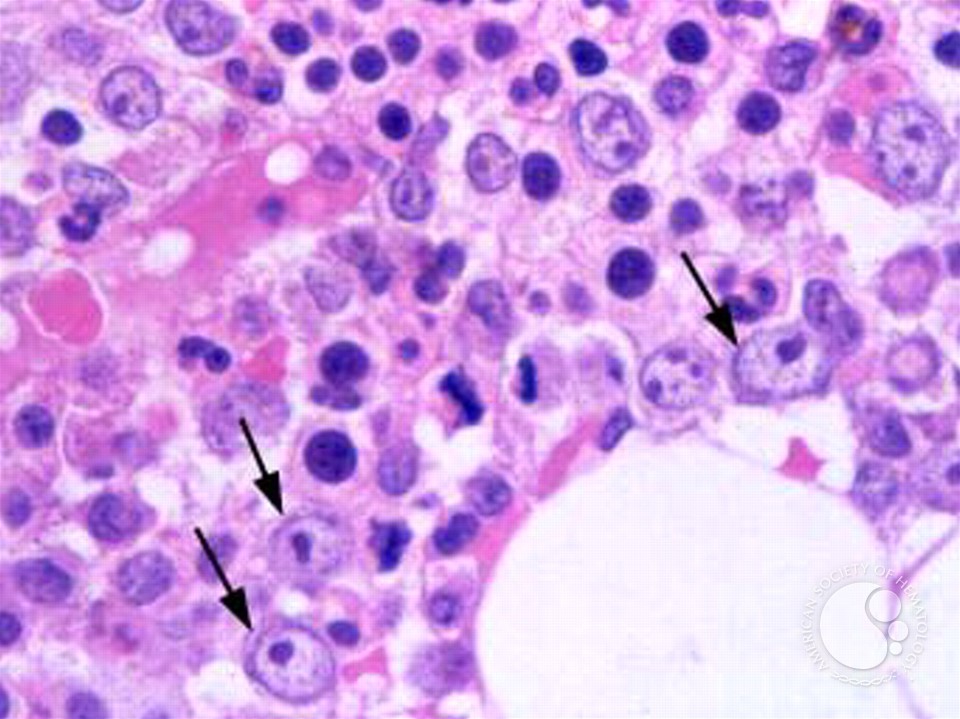
In an analysis of 336 MDS patient samples in Korea, the research team identified the Y259C mutation as a significant factor contributing to a worsened prognosis in MDS patients. Unlike the globally prevalent R525H somatic mutation, Y259C represents an intrinsic mutation specific to East Asian populations, particularly in Korea and Japan.
DDX41, a protein involved in DNA repair processes, plays a pivotal role in maintaining genomic stability. Mutations in DDX41 lead to increased genomic instability through the formation of R-loop structures, where RNA binds to damaged DNA. The RNA within the R-loop undergoes m6A modification, which regulates genomic stability by stabilizing the precarious R-loop structure.
The study revealed a significant increase in R-loops with m6A levels in DDX41 mutant patient samples compared to normal controls, suggesting a failure in proper DNA damage repair mechanisms. Moreover, the research demonstrated how DDX41 mutations disrupt the regulation of m6A-modified R-loops, contributing to the development of leukemia.
Within the R-loop structure, m6A modification is facilitated by the METTL3 and METTL14 complex, with the YTHDC1 protein responsible for recognizing m6A and recruiting DNA repair proteins. Normal DDX41 acts as a crucial mediator, enabling the interaction between the m6A complex and the YTHDC1 protein. However, mutations in DDX41 hinder this essential function, resulting in suppressed recruitment of DNA repair proteins, heightened genomic instability, and potential leukemia development.
Professor Hongtae Kim expressed, “This study provides a comprehensive understanding of the molecular mechanisms of DDX41, a frequently mutated gene in blood cancer. Our findings lay the foundation for the development of strategies to combat leukemia.”
Note:
1. Source: Coherent Market Insights, Public sources, Desk research.
2. We have leveraged AI tools to mine information and compile it.